The Cyclohexane Ring Is Essentially Free of Ring Strain Because
Which microscopic field contains a hypertonic solution answers. Chemistry questions and answers.
Solved The Cyclohexane Ring Is Essentially Free Of Ring Chegg Com
What happens when a rubber balloon is rubbed against wool and gains electrons.

. 5The C-H bonds in cyclohexane are always staggered and never eclipsed in order to reduce there torsional strain. Each of the structures for CIOCI NCOH and CINO has atoms connected in the order given in their formulas. Since 13-diaxal interaction is essentially the steric strain so the larger the size of the substituent the greater the interaction is.
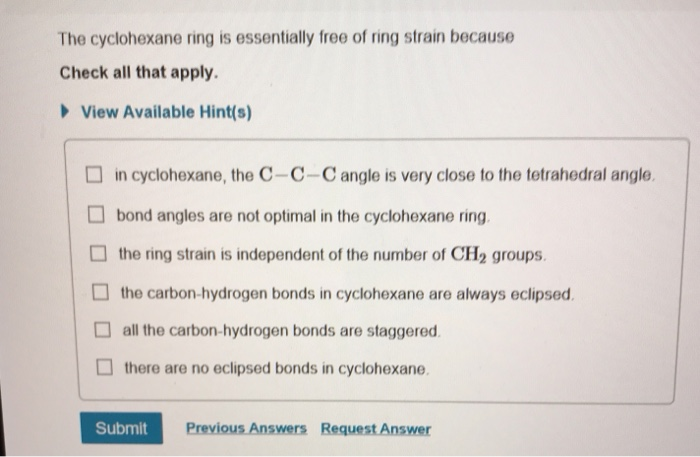
Bond angles are not optimal in the cyclohexane ring. It is quite evident that the cyclohexane ring is completely stable free of the ring strainSo there are no eclipsing bonds present in cyclohexane. The cyclohexane ring is essentially free of ring strain because Check all that apply.
All the carbon-hydrogen bonds are staggered. The carbon-hydrogen bonds in cyclohexane are always eclipsed. This value is low because breaking the.
The cyclohexane ring is essentially free of ring strain because Check all that apply. ANGLE STRAIN arises when the C-C-C bonds of the ring depart because of geometric necessity from the ideal tetrahedral angle preferred for sp3 carbon. The carbon-hydrogen bonds in cyclohexane are always eclipsed.
There are no CH 3 endings because it forms a ring. The cyclohexane ring is essentially free of ring strain because Check all that apply. H2cch2gx2gh2xcch2xg you may want to reference page You serve a volleyball with a mass of 21 kg the ball leaves your hand with a speed of 30 ms the ball has blank energy.
For t-butylcyclohexane the conformation with the t-butyl group in the equatorial position is about 21 kJmol more stable than the axial conformation. They arent exactly 90 because the molecule prefers to take a puckered comformation. As a consequence of its structure cyclopropane has relatively weak C - C bonds DH 65 kcalmol.
The ring strain is independent of the number of CH2 groups. What types of intermolecular forces exist between nh3 and cbr4. Drawing cyclohexane is as simple as drawing a hexagon.
6All the bonds in cyclohexane ring are staggered to eliminate the torsional strain. There are neither eclipsed nor gauche interactions in cyclohexane. If we now look at cyclobutane we see the bond angles are 90.
Cyclohexane has no ring strain due to the fact that it is able to adopt a conformation in which there is no angle or steric strain and minimal torsional strain -this conformation characteristics involve bond angles of approximately 110C and all of the C-C bonds would be staggered. Mgs mgg δh 148 kjmol mgg mg2g What is the concentration m of sodium ions in 457 l. The cyclohexane ring is essentially free of ring strain because Calculate the lattice energy of magnesium sulfide from the data given below.
Identify the type of hybridization. Instead each carbon is connected to a CH 2. The cyclohexane ring is essentially free of ring strain because Check all that apply.
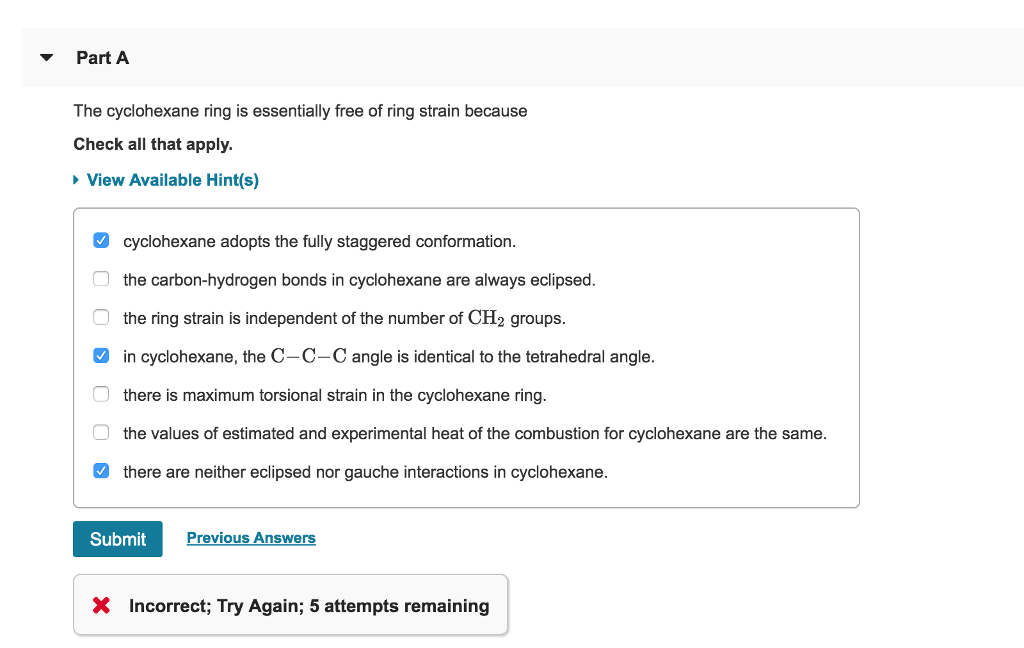
View Available Hint s in cyclohexane the C-C-C angle is very close to the tetrahedral angle bond angles are not optimal in the cyclohexane ring the ring strain is independent of the number of CH2 groups. C 6 H 12 is the chemical formula for cyclohexane. Cyclohexane adopts the fully staggered conformation.
Which statement defines the heat capacity of a sample. The ring strain is independent of the number of CH2 groups. All the carbon-hydrogen bonds are staggered.
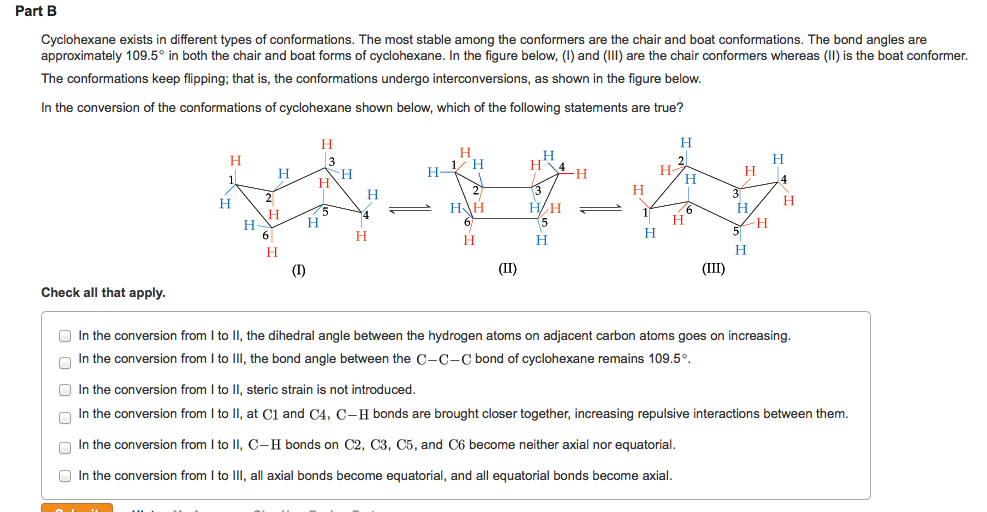
C1 and C4 carbon atoms in the cyclohexane ring are nonplanar. In cyclohexane the CCC angle is very close to the tetrahedral angle. The cyclohexane ring is essentially free of ring strain because.
Ring strain in cycloalkanes also known as angle strain is essentially pent-up energy in the molecule that can be immediately released if the ring is broken. Each point represents a fully saturated with hydrogen atoms carbon in this style. In cyclohexane the CCC angle is very close to the tetrahedral angle.
However it is clear that the bond angles are closer to 1095 than. In addition cyclopropane has considerable torsional strain because the C - H bonds on neighboring carbon atoms are eclipsed. There are no eclipsed bonds in cyclohexane.
The carbon-hydrogen bonds in. Cyclohexane adopts the fully staggered conformation. In cyclohexane the C-C-C angle is identical to the tetrahedral angle.
The carbon-hydrogen bonds in cyclohexane are always eclipsed all the. In the chair conformer all carbon-hydrogen bonds in cyclohexane are staggered with no gauche interactions. Looking at the graph one might think that cycloheptadecane is more stable than cyclohexane because it has a total strain energy of -34 kcalmol.
The cyclohexane ring is essentially free of ring strain because Check all that apply. Notice that the gap in the heat of formation between cycloheptadecane and cyclohexadecane is 10 kcalmol whereas the gap between cyclohexadecane and. Because of the stability difference between the two chair conformers the equatorial-conformation is.
There are neither eclipsed nor gauche interactions in cyclohexane. In cyclohexane the C-C-C angle is identical to the tetrahedral angle. The ring strain is independent of the.
Hence there is no strain in the molecule. Cyclopropane is the most strained of all rings primarily because of the angle strain caused by its 60 C -C -C bond angles. The cyclohexane ring is essentially free of ring strain because.
In cyclohexane the CCC angle is very close to the tetrahedral angle. What is the strongest type of intermolecular force present in chf3. The cyclohexane ring is essentially free of ring strain because Consider the halogenation of ethene where x is a generic halogen.
The cyclohexane ring is essentially free of ring strain because. Bond angles are not optimal in the cyclohexane ring. Again in chair cyclohexane this angle just happens to be virtually identical to the tetrahedral angle so that neither angle nor torsional strain occurs in cyclohexane in the chair form.
The ring strain in cyclopropane is derived from a combination of eclipsing and bond-angle contributions. There is a drop in strain in larger rings.
Solved The Cyclohexane Ring Is Essentially Free Of Ring Chegg Com
Solved Part A The Cyclohexane Ring Is Essentially Free Of Chegg Com

Solved The Cyclohexane Ring Is Essentially Free Of Ring Chegg Com
Comments
Post a Comment